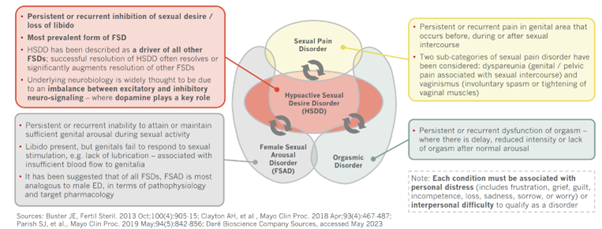
Female sexual dysfunction (FSD) includes a range of issues such as hypo-sexual desire disorder (low libido), difficulty achieving arousal, pain during intercourse, and inability to reach orgasm. Female hypo-sexual desire disorder (HSDD) in the US occurs in 10% of women, independent of age. FSD can profoundly affect the individual’s quality of life and relationships due to the distress, low self-esteem, and anxiety it causes.
There are medical treatment options for young women with FSD, but despite the current options, a large unmet need is to restore the desire for an intimate relationship with the partner. Initiator Pharma will investigate the potential for its products with a priority on postmenopausal women with FSD, where there currently is no available treatment option.
Pudafensine and IP2018 offer the potential as first-line treatment options in postmenopausal generalized, acquired HSDD – where it would be positioned as the first approved therapy. Both products offer the potential of clear differentiation from current FSD drugs, with the key differentiators:
- Non-hormonal mechanism of action
- Clean safety/tolerability, no drug interaction or contraindication issues (as shown in completed trials in men with Erectile Dysfunction)
- Convenient, oral, on-demand dosing
- Potentially improved efficacy to Addyi and Vyleesi (currently only approved for use in HSDD in premenopausal women)

